-
Commercial Of: +51 1 241 5659
Diagnostic Imaging Center:
+51 990 046 244/+51 1 379 9810 - Frequent questions
ING
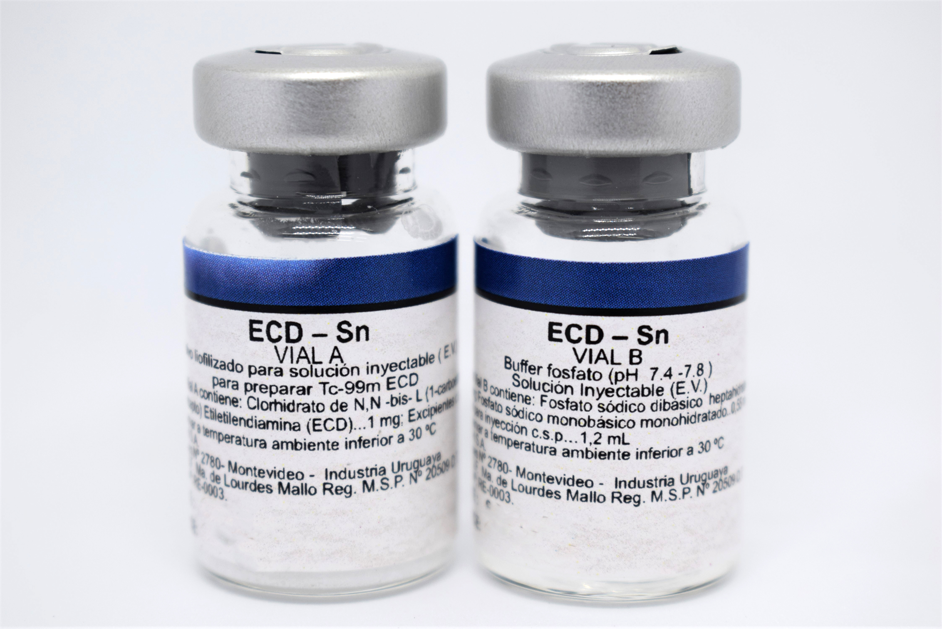
Qualitative and quantitative composition
VIAL A
Contains lyophilized, sterile, vacuum sealed:
| N, N-bis-L (1-carboethoxy-2- mercapto)Ethyleylenediamine hydrochloride (ECD) |
1.0 mg. | |
| Mannitol | 20.0 mg. | |
| Na2 EDTA, dihydrate | 0.36 mg. | |
| Stannous chloride dihydrate | 90.0 µg. |
VIAL B
| Contains in sterile liquid form: Buffer phosphate pH 7.4 – 7.8 |
1.2 mL. | |
Pharmaceutical form: lyophilized powder for injectable solution
Description and indication
ECD-Sn has no indications by itself.
After reconstitution with Tc-99m, it is used in brain perfusion studies and for the vascular
diagnosis of cerebral alterations.
99mTc-ECD in Single photon emission computed tomography (SPECT) is indicated as an
adjunct to conventional CT tomography or magnetic resonance imaging MRI in the location of
cerebral infarction in patients in whom they have already been diagnosed.
This medicinal product is for diagnostic use only